Chapter 8 Physical and Chemical Properties of Soil
Soil is not inert. It is a mix of organic and inorganic particles, each with different physical and chemical properties. Micro-organisms and macro- invertebrates like worms further alter its properties. Rather than try to monitor every possible variables, professional growers monitor these main traits.
- Relative air/water space (porosity)
- pH
- Fertility
- Cation exchange capacity
- Total soluble salts (electrical conductivity)
- Wettability
Together these factors provide a well-rounded view of how a particular type of soil (or a soil-less mix) will affect plant and tree growth and health.
8.1 Relative Air/Water Space, or Porosity
Both natural soils and soilless mixes have spaces between the organic and inorganic particles called voids that hold air and water. Soil porosity (n) is the ratio of the volume of voids to the total volume of the soil:
n = Vv / Vt
Where Vv is the volume of the voids (regardless of whether they are filled with water or air), and Vt is the total volume of the soil.
Overall soil porosity depends on particle sizes of the soil or soil-less mix and how tightly they are packed. All other factors being equal, the more porous soil is, the faster water enters and passes through, the more air is available for roots and the faster the material dries out. Smaller particles pack more tightly and hold more water (and so more nutrients), at the cost of reducing the available air.
When soil is compacted, most of the pore spaces have collapsed so water and air cannot get in as easily. In open ground, foot traffic is the most common cause of compaction. Soil in pots gets compacted through watering, by normal breakdown of the organic material, if the soil mix is packed too tightly, or as roots grow into the spaces.
Understanding soil porosity helps us understand which materials are most appropriate for making soil mixes. Cornell University Extension has published a general rule of thumb that a good container mix should have total porosity >50% overall, moisture holding capacity >40%, and air porosity >10%. A typical bonsai soil mix can be slightly below that target, with an overall porosity of 40-45%.
Clemson University Extension reports that southern pine bark milled to the size range appropriate for bonsai soil mix has ~82% overall porosity, ~44% moisture holding capacity, and ~38% air porosity. What inorganic materials would be the best choices for creating a good bonsai mix on a consistent basis?
Let’s assume we need mixes ranging from 4 parts bark and 1 part inorganics to a 1:1 mix. To keep this example simple, we’ll just calculate overall porosity. The table below lists porosity of different types of inorganic materials. Which would be reasonable additives, and which ones would be risky to use?
| Description | Overall Porosity Range (min - max) | Average | Range |
|---|---|---|---|
| Silty gravel | 15-22% | 19% | 7% |
| Coarse sand | 26-43% | 35% | 17% |
| Fine sand | 29-46% | 38% | 17% |
| Graded gravel or sandy gravel, w/o fines | 21-38% | 30% | 19% |
| Clayey sands | 15-37% | 26% | 22% |
| Silty sands | 25-49% | 37% | 24% |
| Inorganic clays | 29-59% | 44% | 30% |
| Inorganic silts | 21-56% | 39% | 35% |
| Silty or sandy clay | 20-64% | 42% | 44% |
(Data source: http://geotechdata.info/parameter/soil-void-ratio.html)
Silty gravel has lots of small particles and is likely to fill in the pores, so is not the best choice. Fine sand, coarse sand and graded gravel provide good porosity across the full range of mixes we want (I used 34% as an average value for the three):
4:1 bark:inorganics = (82*4)+34 = 362/5 = 72% porosity 3:1 bark:inorganics = (82*3)+34 = 280/4 = 70% porosity 2:1 bark:inorganics = (82*2)+34 = 198/3 = 66% porosity 1:1 bark:inorganics = (82*1)+34 = 116/2 = 58% porosity
It would be possible to use inorganics that are mostly silt or clay, but they would create problems. First they all have small particles that are going to collapse the soil structure quickly. Second, they have a very wide range of porosity depending on the source. This would make it hard to create soil mixes consistently. It is simpler just to stick with using coarse sand and gravel for the inorganic phase.
8.2 pH
Soil pH is a measure of relative acidity or alkalinity of the soil. pH is described by a log scale in which 7 is neutral for soils measured in water. Soils of pH:
- Lower than 3.5 are unusual and generally toxic to plants
- Less than 4.5 as strongly acid
- 4.5 to 5.5 as acid
- 5.5 to 6.5 are slightly acid
- 6.5 to 7.5 is considered neutral
- Above 7.5 is alkaline
General soil pH guidelines are:
- pH 5 to 5.5 for ericaceous species (azaleas), Vaccinium (blueberries and their kin), and camellias.
- pH 5.5 to 6 for conifers. Conifers tend to become chlorotic on soils of neutral or alkaline pH because of their inability to obtain adequate Fe and Mn.
- pH 6 to 6.5 for hardwoods, junipers, maples, and arborvitae.
Most plants grow best in pH 5.5–6.5, although some plants are particularly tolerant of acidity (for example Inga edulis, Casuarina junghuhniana) or alkalinity (for example Prosopis chilensis, Tecoma stans).
8.2.1 Why is pH Important?
Nutrients become more or less bio-available for plants at different pH levels. pH also influences the composition of soil flora and fauna, including some crop pathogens.
- Nitrogen (N), potassium (K), calcium (Ca), and magnesium (Mg) are most readily available at soil pH >6.
- Phosphorus (P) is only bioavailable between pH 6-7.
- Metal micronutrients including iron (Fe) , manganese (Mn), zinc (Zn), copper (Cu), and cobalt (Co) more available for uptake at pH values below about 5.5.
- Extremely acid soils (pH < 4.5) are infertile because they do not retain cations such as NH4+, K^+, and Ca^+2 to any extent.
- Incidence of damping-off is reduced when nursery soil pH is maintained in the region of 4.5 to 6.0.
- Weed growth is reduced by acid soils.
8.2.2 What Determines Soil Mix pH?
Most soil-less mixes contain organic components (like peat or pine bark) that are naturally acidic. As substrate components are mixed they affect pH differently, making it hard to predict final pH of of a soil-less mix. Fertilizers and other additives can further change the pH. For example:
- Ammonium (especially ammonium sulfate) and urea salts make soil more acidic.
- Potassium of calcium nitrate fertilizers (KNO3 or Ca(NO3)2) raise soil pH.
- Phosphate-containing fertilizers either have no effect on soil pH or raise it. The exception is ammonium phosphate, which reduce soil pH.
- Potassium sulfate and chloride have negligible effects on soil pH.
- Aluminum sulfate reduces soil pH.
8.2.3 Raising Media pH
Agricultural/dolomitic limestone is a combination of calcium carbonate and magnesium carbonate. It raises pH of the growing medium but also provides some additional magnesium and calcium for plant uptake. Dolomitic limestone dissolves slowly in the growing medium, stabilizing pH for several months.
There is no simple way to calculate precisely how much limestone you will need to add to reach a desired pH. It depends on the current pH and cation exchange capacity of the media (which in turn depend on your source of organic materials) and the particle size of the limestone. The only way to be sure how limestone affects pH is through trial and error.
As a general guide, commercial growers use between 2-8 pounds of dolomitic lime/cubic yard of soilless media. Other references say that 7-14 cups/cu.yd. are needed to adequately buffer pH. When in doubt, make a test batch of soil with no limestone added. Check the pH, then add HALF as much limestone as you think is needed, or on the low end of the stated reference range. Recheck the pH, and add more limestone if needed to reach the desired pH.
Keep in mind that it is hard to lower soil mix pH if you add too much lime. It is better to add too little, check the mix pH, then add more. Once you have made a few batches of soil, you will have a good idea of how much limestone is needed for the bulk materials you use in your mixes.
When buying dolomitic limestone, look for 100-mesh/fine grade. The more finely ground 200-mesh/superfine grade changes pH more quickly, but does not keep the soil pH stable for as long.
Instructions for testing soil pH at home are located in the final section of this page.
8.2.4 Lowering Media pH
It is unusual to need to lower soil pH. Generally, it is only necessary when your water source is hard or alkaline. To lower the pH add aluminum sulfate, ferrous sulfate, or chelated iron. Using ammonium-based fertilizers can lower the media pH over time, as can liquid fertilizer formulated for acid-loving plants.
8.3 Fertility
Fertility is a catch-all term for nutrient availability. As soon as a seedling has used up the nutrients stored in the seed, it needs to start taking in nutrients from the growth medium. There are three macronutrients that plants need in relatively large amounts: nitrogen (N), phosphorus (P) and potassium (K). Plants also need smaller amounts of other micronutrients: calcium, magnesium, iron, manganese, zinc, copper, boron, and molybdenum. Macro- and micronutrient deficiencies produce very specific symptoms; see Identifying Nutrient Deficiencies for more details.
One of the main measures of soil fertility is the carbon:nitrogen (C/N or C:N) ratio of the organic matter in the soil. Carbon in soil is the primary source of energy for microbial life, while nitrogen is used by microbes for making amino acids and nucleic acids. These microbes in turn regulate availability of nitrogen and other macro- and micronutrients to tree roots.
Balanced soil will have a C:N ratio in the range of 15:1 and 35:1. If the C:N ratio goes higher than 35:1, the extra energy stimulates microbial growth. To make proteins and nucleic acids, these microbes must remove bio-available nitrogen ions from the soil. This process, called nitrogen draft or nitrogen draw down, reduces the amount of nitrogen available to plant roots. This can cause serious yellowing and stunting of trees.
C:N ratio is particularly important for soil-less mixes because they are made with organic materials like pine bark, peat, or coir. Fresh pine bark may have a C:N ratio of 300:1, while sawdust can be as high as 1000:1. To compensate and limit potential nitrogen draft, commercial growers will add 0.25 to 1 pound N/yd^3 to fresh bark. Ideally this will be in a slow-release form like blood or soybean meal rather than as chemical fertilizer.
8.4 Cation Exchange Capacity (CEC)
If soil was made up of inert particles, it could not hold nutrients; the soluble nutrients would be leached out and washed away every time we water. Cation exchange capacity (CEC) is the ability of negatively charged particles in soil or soil-less mixes to adsorb positively charged ions (‘cations’) and hold them in place. These nutrients are stored on growth medium particles until they are taken up by the root system. Cations that participate in this process include calcium, magnesium, potassium, ammonium, iron, manganese, zinc and copper.
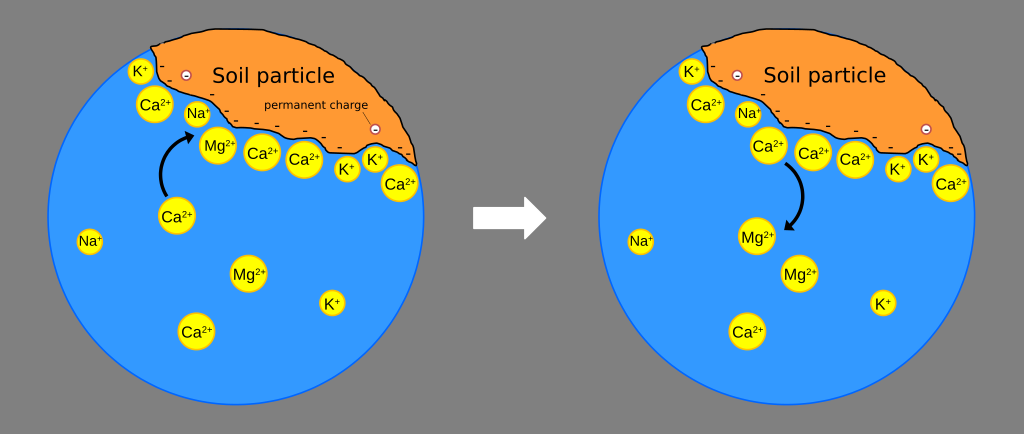
The ions that participate in soil cation exchange. Link to original image.
Cations adsorbed to clay micelles and organic matter can be displaced by other cations that are more positively charged or as a result of mass action. The quantity of cations which can be adsorbed or displaced is the cation exchange capacity (CEC) of the soil. This measurement is important for soil fertility because nutrient cations held on the soil CEC are not leached but are available for plant growth. Although CEC is normally measured at pH 7, nursery soils may have lower pH values and high organic matter contents such that the effective CEC may be lower than the measured CEC.

Cations and pH
How pH affects cation binding. Link to original image.
CEC is measured in milliequivalents (meq), which describes binding capacity of soil for nutrient cations. The meq values are normally expressed on the basis of 100 g dry soil. For example, a soil with a CEC of 20 meq could adsorb 20 meq Ca, which equals 400 mg Ca (=20 meq x mol. wt. of Ca (20.0 g)), 20 meq K (=782 mg K), or 20 meq Mg (=243 mg Mg).

CEC measurement
How CEC is measured. Link to original image.
Some soils contain high amounts of clay that absorb cations so strongly that they become unavailable for plant nutrition (mineral ‘fixation’). This is why clay is not added to nursery mixes, even though it holds nutrients well.
Although the CEC of some soil-less substrates is very high, anions still get washed out easily and need to be replenished. This is particularly important for phosphorus and for nitrogen in the form of nitrate. Mixing a slow-release P-rich fertilizer, such as rock phosphate, into the substrate before planting can help alleviate this problem.
8.5 Electrical Conductivity
Electrical conductivity (EC) is a good estimate for the total soluble salts in a media. Salts in the form of fertilizers and essential minerals are needed for tree growth, but excessive fertilizer (alone or combined with salts from other outside sources) prevent normal water and nutrient uptake in the roots. Growth is stunted and leaf tips scorch.
Salt sensitivity depends on age and species of tree. In general, soil-less mixes with an EC in a range of 2.0-3.5 dS/m are safe for all but salt-sensitive species. Azaleas, camellias, Ficus benjamina, and most seedlings do better in media that is between 0.75 and 2.0 dS/m. Soil with an EC <0.75 dS/m may not have enough nutrients to support growth.
If salts are too high, the first objective is to determine the source. Usually it will be from the irrigation water or from over-fertilization. Once the source is identified, you will want to determine if you can reduce or eliminate that source.
High salt levels in potted trees can be corrected by flushing trees with enough low salinity water that 25-30% of the total volume of the container runs off. For example, if you are trying to flush a 10-gallon container, over-water enough that 2-3 gallons of water runs out of the container each time. Return to normal watering and less frequent fertilization once the soil EC returns to normal.
8.6 Wettability
Peat-based soil mixes are susceptible to drying out then not re-wetting. Permanently dry spots in pots develop and reduce effective soil volume. Wetting agents (surfactants) can be added to irrigation water, but are not ideal for routine use.
It is better to pre-check a new batch of soil mix by watering a small pot containing prepared nursery mix by itself, waiting 30 minutes, then pouring it back out.
If the mix is evenly wet, watering is sufficient without changes. If the mix has a wet top layer and bottom, but dry patches, more water is needed per pot. If the mix has obviously wet channels that funnel water away from the dry mix, the peat or bark is not wetting properly. Consider pre-wetting it with water mixed with a wetting surfactant before using the mix.
8.7 Measuring Physical and Chemical Properties of Soil
8.7.1 Soil Conductivity and pH
The standard protocol for testing EC is to make a 1:1 volume ratio of distilled water and soil. Tamp down one sampling scoop of soil by tapping it carefully on a hard level surface. Place the soil in a mixing container, then add 1 sampling scoop of distilled water. Cap the mixing container and shake 10-15 seconds. Insert the EC probe into the soil-water mixture, keeping the probe tip well in the center area of the soil suspension. Take the reading while soil particles are still suspended in solution. When reading stays stable for ~10 seconds, record the EC1:1 value in dS/m. Turn off and thoroughly rinse the EC probe with distilled water and replace the cap.
There are two ways to measure soil pH. Most agricultural soil conductivity meters also measure soil pH. Alternatively you can measure pH using Hydrion paper test strips. They are simple dip sticks that you can purchase from Amazon, A.M. Leonard, Forestry Supply, or science supply companies.
Figure 1. Paper pH test strips. Figure 2. A pocket pH tester. Link to original image 1;image 2.
The same soil-water mixture can be used to measure nitrate and phosphorus levels if kits are available.
8.7.2 Overall Fertility and CEC
There are no methods for measuring either fertility or CEC at home. Soil tests must be done by the state lab. You can submit samples for testing through the local Cooperative Extension Office.

