Chapter 9 Macronutrients, Micronutrients, and Fertilizers
9.1 Fertilizers For Container Production
All container-grown plants need to be fertilized regularly, because soil-less mixes contain very few nutrients of their own. Fertilizer may either be a liquid, dry granules, or slow release granular material. Professional nurseries often use a combination of both dry and water soluble fertilizers.
For a smaller number of trees, water soluble fertilizers can be mixed and applied between regular watering. For a larger number of trees, injecting pre-mixed concentrated fertilizer into routine irrigation water (ferti-gation) can be more efficient.
Dry granular materials can be mixed into the soil mix prior to potting or applied to the substrate surface (top-dressing). Simple granular fertilizers do not last an entire growing season and may need to be re-applied around midsummer. Controlled-release fertilizers (CRFs) have largely replaced plain granular fertilizers in nursery production. These products provide more consistent nutrient availability to plants over time than traditional granular fertilizers. Nutrients diffuse slowly from “capsules” of resin or polymer at a rate that depends on moisture content and temperature of the substrate. CRFs are available in several formulations to suit the nutrient requirements of different plants, and with different coatings to provide nutrient release over different time frames (e.g. 4, 8, or 12 month formulations).
9.2 Macronutrients and Macronutrient Deficiencies
These are the nutrients that plants transport out of the soil and use in large quantities. Routine fertilization is essential for healthy tree growth because it provides these nutrients consistently.
Nitrogen (N) is needed for making chlorophyll, proteins, and other molecules a plant needs for vegetative growth. Applying nitrogen triggers a spurt of rapid growth, especially of parts that are above ground. It is important to apply high nitrogen fertilizer only when a tree is actively growing. In spring, wait until AFTER growth has started, or new leaves have emerged following leaf pruning. Then apply a balanced, high nitrogen fertilizer to replenish the treeʼs resources. If the foliage is dull a little extra nitrogen may improve the color.
High–nitrogen fertilizer can also be used to force young, developing stock plants to grow to training size more quickly. In this case, the young tree is fed more frequently than a mature specimen.
Nitrogen deficiency (Image 1) and over-use/burn (Image 2). Link to original image 1, by John Ruter, University of Georgia, Bugwood.org .; Link to original image 2, by Lacy L. Hyche, Auburn University, Bugwood.org..
Phosphorus (P) is needed for growth of healthy roots. A little extra phosphorus is especially useful after repotting or when a tree is recovering from a root problem. Reducing nitrogen and increasing phosphorus and potassium (described below) in late summer and autumn helps trigger a treeʼs preparation for dormancy. The roots toughen and top growth slows in preparation for winter. Buds harden and scale over, and the sap flow slows. Lack of phosphorus is hard to spot, but often plant growth is slow, and the stems of green shoots develop a purple tinge.

Purple discoloration of grape leaves is a diagnostic feature of phosphorus deficiency. Link to original image.
Potassium (K) is needed by all plants, but is especially important for flowering and fruit formation. Unlike our clay soil, bonsai soil does not hold on to potassium well, and it will leach from a pot during regular watering. Azaleas and other flowering species are particularly susceptible to potassium deficiency. Symptoms often start in older parts and lower leaves, then move upward towards younger tissues. Common signs are yellow scorching of the edges of leaves, moving into the leaf centers, followed by leaf drop.

Potassium deficiency. Link to original image, Daren Mueller, Iowa State University, Bugwood.org..
9.3 Micronutrients and Micronutrient Deficiencies
Even with regular fertilization, trees occasionally develop micronutrient deficiencies. Micronutrients assist the enzymes that produce sugars, help build chlorophyll, and drive thousands of other plant processes. Most micronutrient deficiencies cause dramatic changes in the color and shape of leaves. For example, lack of iron causes the veins of leaves to remain green, but the spaces between them to turn yellow. In contrast, insufficient sulfur causes the entire plant to yellow, and the veins to turn pink or red. Brown, curling leaves in the absence of insects or fungus suggests micronutrient deficiency too.
Trees need small amounts of six elements for the growth and development:
- Iron (Fe)
- Manganese (Mn)
- Copper (Cu)
- Zinc (Zn)
- Molybdenum (Mo)
- Boron (B)
There are other micronutrients, but they are required in such small amounts that it is virtually impossible to develop a deficiency outside of a lab setting.
I’ve adapted these sections from Micronutrient Deficiencies, an article written by E. Thomas Smiley, Ph.D., Plant Pathologist with Bartlett Tree Research Labs, Charlotte, NC.
9.3.1 Iron
Iron is required for chlorophyll production, and thereby, for photosynthesis. Iron also forms a complex with sulfur that is used in photosynthesis. Additionally, iron is required by enzymes that transfer and release energy, and strengthen cell walls.
Lack of iron causes yellow leaves due to low levels of chlorophyll. Leaf yellowing first appears on the younger upper leaves, between the veins, a condition called interveinal chlorosis. Severe iron deficiency makes leaves turn completely yellow or almost white, and then brown as leaves die.
Iron deficiency happens mainly on high pH soils, and sandy soil with little organic matter. Cool, wet weather and poorly aerated or compacted soils also reduce iron uptake by plants.
Interveinal chlorosis, a symptom of iron deficiency. Link to original image.
9.3.2 Manganese
Manganese (like iron) is essential in photosynthesis. It also is necessary for the plant to use nitrogen properly.
Like iron deficiency, too little manganese causes interveinal chlorosis. In very severe cases, brown dead spots appear on leaves, and leaves drop prematurely.
Like iron, manganese deficiencies are most common in high pH soils.
9.3.3 Copper
Copper is needed for carbohydrate and nitrogen metabolism, so inadequate copper results in stunting. It also is required for making lignin that strengthens cell walls and promotes strong wood formation.
Too little copper causes dieback of stems and twigs, yellowing of leaves, stunted growth and pale green leaves that wither easily.
Deficiencies happen in sandy soils which are low in organic matter. Copper uptake is blocked by high soil pH, or if there is too much phosphorus (P) in the soil.

Copper deficiency. Link to original image, John Ruter, University of Georgia, Bugwood.org..
9.3.4 Zinc
Zinc is an essential component of various enzymes for energy production, protein synthesis, and growth. Deficiency symptoms are short internodes and leaves that are smaller than normal. Zinc is unusual in that it does not move around in phloem sap of, meaning zinc-deficiency symptoms occur mainly in new growth.
Deficiencies are mainly found on sandy soils low in organic matter and on highly organic soils. Zinc deficiencies occur more often during cold, wet spring weather, when roots are working more slowly, and the microbes that release zinc from organic matter are not as active.
Like most micronutrients, zinc becomes less available if soil pH is alkaline.

Zinc deficiency in pecans. Compare the sizes and symmetry of the leaflets in the leaves. Link to original image, University of Georgia Plant Pathology , University of Georgia, Bugwood.org..
9.3.5 Molybdenum
Molybdenum is used by enzymes that control nitrogen use, protein synthesis and sulfur metabolism. Molybdenum requirements are so low that most plants do not exhibit symptoms. Deficiency usually looks like nitrogen deficiency instead.
9.3.6 Boron
Boron is used for cell wall formation, so boron-deficient plants may be stunted. It is also required for sugar transport. Lack of boron first appears at the growing points.
Boron deficiency is found mainly in acid, sandy soils, areas of heavy irrigation, and soils with low organic matter. Borate ions are mobile so can leach from the root zone easily. Boron deficiency is more pronounced during drought periods when root activity is slower.

Boron deficiency on crabapple leaves. Link to original image, University of Georgia Plant Pathology.
9.3.7 What Causes Deficiencies?
Several factors contribute to macro- and micronutrient deficiencies. Alkaline soil pH is the most common factor predisposing trees to micronutrient deficiencies. It can happen in soils with a pH as low as 6.3 but is more common when the pH is above 7.0. With high pH, micronutrients are converted to chemical forms that certain plants cannot absorb. In sandy soils, the critical nutrient may simply be lacking. In heavier soils, too little or too much water may reduce nutrient uptake resulting in deficiency.
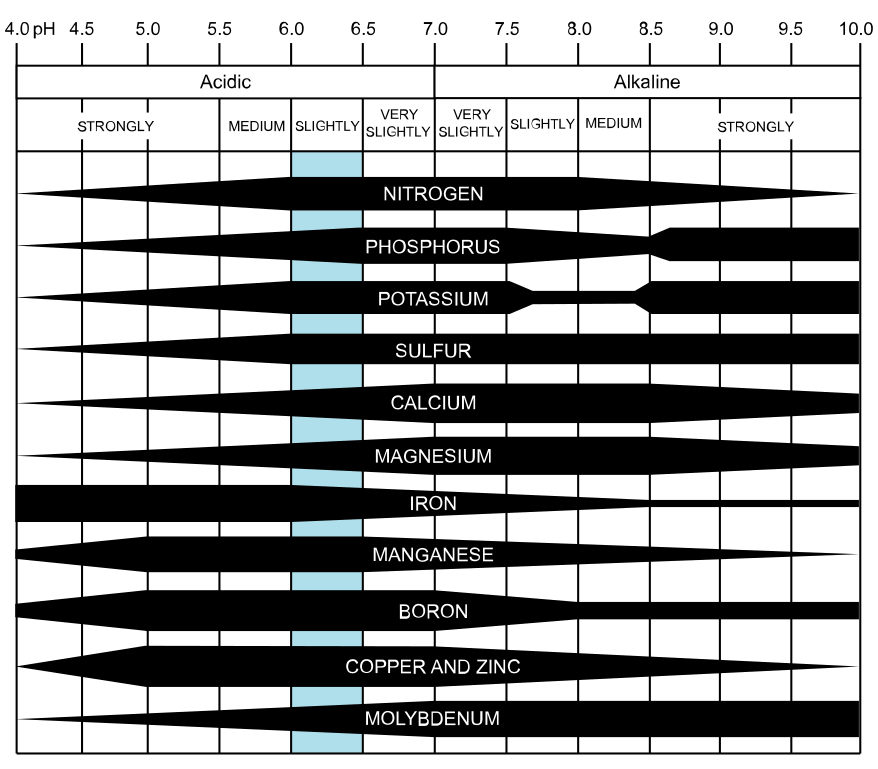
Effects of soil pH on nutrient availability. Link to original image
In bonsai soils micronutrient deficiencies are most often caused by under-fertilization, using fertilizers without micronutrients added, over-watering, or excessive rainfall.
9.3.8 Diagnosing a Deficiency
To me, these descriptions all sound about the same. Moreover, the same deficiency can look different between species. This makes diagnosis harder still. Many experts recommend using color photos to distinguish between deficiencies. Search for photos online rather than using books, so you have more to compare.
Diagnosis is made easier by the fact major groups of plants tend to respond similarly. We can limit ourselves to the effects on woody landscape plants (temperate bonsai), and woody houseplants (tropical bonsai).
According to Dr. Smiley, iron and manganese deficiency are the most common in woody landscape trees and shrubs. The main trees susceptible to iron and/or manganese deficiency are:
- Oaks (esp. pin, white)
- Azaleas, rhododendrons
- Japanese black pine
- White pine
- Birches
- Maples
- Flowering cherry, other Prunus
- Crabapple, other Malus
Interveinal chlorosis is the earliest symptom. Severe deficiencies kill leaves, stunt growth and cause premature defoliation. Lack of photosynthesis can lead to twig dieback, especially at the top of the tree, and branch dieback if the condition persists. With iron deficiency the fine veins in chlorotic leaves are often green. With manganese deficiency the fine veins become chlorotic too.
Tropical trees tend to grow in more organic–rich soil, which can break down and compact or become waterlogged. They also are watered more frequently. As a result, tropicals are likely to develop boron deficiency, in addition to iron and manganese deficits.
9.3.9 Treating Deficiencies
For trees in pots, usually the soil is simply low in the needed nutrient. If the soil pH is below 6.0, adding a micronutrient fertilizer should be sufficient. Useful fertilizers include Milorganite for iron, iron sulfate or manganese sulfate (not magnesium sulfate (Epsom salt)). If the pH is 6 to 7, a chelated nutrient like Liquid Iron is a better choice, since chelation prevents iron’s conversion to insoluble forms by high pH.
If the soil pH is alkaline (above 7) the soil pH must be reduced by applying sulfur. Another option is to feed the tree for several weeks using the acidic formulation of Miracle–Grow or Peter’s Plant Food. Both products will lower the soil pH and make micronutrients available again. They also contain the necessary micronutrients.
If tropicals do not respond, soil quality or compaction may be the problem. Consider repotting to better soil, particularly if a tree has been in the same pot for some time.
For trees in the ground, the problem may be a little more difficult to correct. Start with a soil test of your growing area; the forms and instructions are available for free at the County Extension Office. Also ask them to conduct a micronutrient analysis. When you get the results, look at the soil pH. If the soil pH is 5–7, use the same methods as for potted trees. If it is above 7, use sulfur to reduce the pH.
Several applications of micronutrients may be required to correct the problem in a large area. If the soil analysis indicates the soil is calcareous (i.e., alkaline and contains excessive lime), it will be practically impossible to adjust the soil pH. The best strategy is to dig up your trees, remove the alkaline soil, and replace it.
9.4 How to Read Fertilizer Labels
Fertilizers are required to have the concentration of macronutrients listed on the label. The N-P-K ratio will be listed by numbers like 10–10–10, 6-2-4, or some other ratio. The numbers indicate the concentration of macronutrients as a percent of the total weight of the container. For example, a 10-pound bag of 10-10-10 fertilizer will have 10 pounds x 10% = 1 pound each of nitrogen, phosphorus and potassium. A 10-pound bag of 6-2-4 will contain 0.6 pounds of nitrogen, 0.2 pounds of phosphorus, and 0.4 pounds of potassium.
1. 
2. 
3. 
4. 
How to read a fertilizer label. These are photos of the back of a 50 lb bag of controlled release fertilizer I use. Image 1. Text. Image 2. text. In addition to its composition, many containers provide helpful information about use rates. Image 3. Normal rates at which this fertilizer would be mixed into container soil mixes. Image 4. Normal amounts used for top-dressing (adding to the soil surface.) Original photos and annotations by Dan Johnson.
Unfortunately there are no standards for listing micronutrients on the label. The only way to ensure your fertilizer has the essential micronutrients is to look through the list of components in the mix.
In addition to a complete fertilizer plus micronutrients, additional dolomitic lime often is added to soil mix to increase the pH and to provide an additional source of calcium and magnesium.
9.5 Checking the Effectiveness of Fertilization
Regardless of your fertilizer sources and formulation, it is a good idea to check the nutrient levels in random samples of trees in order to make adjustments before any problems develop. An easy method to use is the “pour through” method that was developed at Virginia Tech. Details of the pour-through method of monitoring nutrients in containers can be accessed at: http://www.ces.ncsu.edu/depts/hort/nursery/cultural/topic4.htm


